L'invecchiamento è qualcosa che tutti noi attraversiamo e gli scienziati sono sempre stati curiosi al riguardo. Con il passare del tempo, il nostro corpo non funziona più bene come prima. Ciò significa che potremmo ammalarci più spesso o impiegare più tempo per guarire. Alcuni di noi potrebbero addirittura affrontare problemi di salute più gravi, come le malattie cardiache o il cancro. Un famoso studio chiamato "The Hallmarks of Aging" ha elencato nove ragioni principali per cui invecchiamo. Poi, nel 2022, i ricercatori di Copenhagen hanno aggiunto altri cinque motivi a quell'elenco. Tutte queste ricerche ci aiutano a comprendere meglio i cambiamenti che avvengono nel nostro corpo quando invecchiamo.
In Purovitalis, siamo profondamente impegnati nella comprensione di questi tratti distintivi dell'invecchiamento. Crediamo che, concentrandoci su di essi, possiamo creare integratori che supportino veramente il nostro corpo durante l'invecchiamento. Ma sappiamo anche che la scienza può essere complicata. Ecco perché ci impegniamo a spiegare questi segni distintivi in un modo che sia facile da capire per tutti. Il nostro obiettivo è quello di colmare il divario tra i concetti scientifici complessi e la conoscenza quotidiana, assicurando che lei sia ben informato e sicuro delle scelte che fa per la sua salute.

Diamo un'occhiata più da vicino a questi 14 segni distintivi per capire meglio cosa succede nel nostro corpo durante il processo di invecchiamento:

Pensi al suo DNA come al manuale di istruzioni del suo corpo. L'instabilità del genoma può offuscare queste istruzioni a causa di errori di replicazione del DNA o di stress cellulare. Anche se i danni minori al DNA non sono sempre dannosi, possono accumularsi nel tempo, causando il malfunzionamento delle cellule e contribuendo all'invecchiamento.
Se una cellula presenta troppi errori, soprattutto nei mitocondri (i produttori di energia della cellula), potrebbe non funzionare in modo efficiente. Tuttavia, sostanze come il resveratrolo, il magnesio, la vitamina B, la melatonina, la creatina e l'NMN possono sostenere una sana funzione mitocondriale.
Pensi al suo DNA come a un laccio da scarpe, con i telomeri che assomigliano alle piccole punte di plastica alle estremità. Questi telomeri proteggono il nostro DNA essenziale.
Perché i telomeri sono associati all'invecchiamento?
I telomeri impediscono al nostro DNA di attaccarsi ad altri componenti cellulari. Se il DNA è il manuale di istruzioni del corpo, i telomeri assicurano che queste istruzioni rimangano separate e non danneggiate. Inoltre, regolano la frequenza con cui una cellula può dividersi. Quando le cellule si dividono, i telomeri si accorciano, agendo come un conto alla rovescia. Quando si accorciano troppo, la cellula smette di dividersi, il che può portare all'invecchiamento o al danneggiamento della cellula. Alcuni ricercatori ritengono che questo processo possa essere legato all'invecchiamento umano. Preservare i telomeri potrebbe potenzialmente prolungare la nostra durata di vita.
I cambiamenti epigenetici sono come interruttori che possono accendere o spegnere determinate parti del nostro DNA. Non cambiano il DNA stesso, ma possono influenzare il suo funzionamento. Questi cambiamenti possono essere influenzati dalla dieta, dallo stress e dall'ambiente in cui viviamo.
Quando invecchiamo, questi interruttori possono influenzare aspetti come l'infiammazione e il modo in cui le nostre cellule producono energia. Se troppi interruttori sono nella posizione sbagliata, l'invecchiamento può essere più rapido.
Comprendendo e gestendo questi interruttori, potremmo essere in grado di invecchiare in modo più sano. Semplici cose come mangiare bene, ridurre lo stress ed evitare abitudini dannose come il fumo, possono aiutare a mantenere questi interruttori nella giusta posizione.
Con l'avanzare dell'età, il nostro corpo potrebbe faticare a tenere sotto controllo le proteine, causando problemi come infiammazioni e problemi agli organi. Se si accumulano troppe proteine danneggiate, si può accelerare l'invecchiamento e causare malattie.
La buona notizia? Possiamo aiutare il nostro corpo a gestire le proteine. Mangiare meno, digiunare e seguire diete come la Mediterranea o la DASH possono aiutare. Inoltre, è essenziale vivere una vita senza stress, perché lo stress può alterare il nostro equilibrio proteico e la nostra salute generale.
Un recente studio del 2022, condotto a Copenhagen, ha evidenziato un nuovo aspetto dell'invecchiamento. I piccoli organismi del nostro intestino, noti come microbioma intestinale, subiscono dei cambiamenti nel corso del tempo. C'è un cambiamento nel tipo di microbi e una diminuzione della varietà di specie. Inoltre, le barriere del nostro corpo, come la parete intestinale, possono indebolirsi, provocando infiammazioni. Queste alterazioni della salute dell'intestino possono provocare un'infiammazione che influisce sul nostro benessere generale.
Mantenere un intestino sano può comportare cambiamenti nello stile di vita, come mangiare grassi sani, bere molta acqua e rimanere attivi.


*Queste affermazioni non sono ancora state stabilite ufficialmente dalle autorità (europee) e si basano su articoli di ricerca.

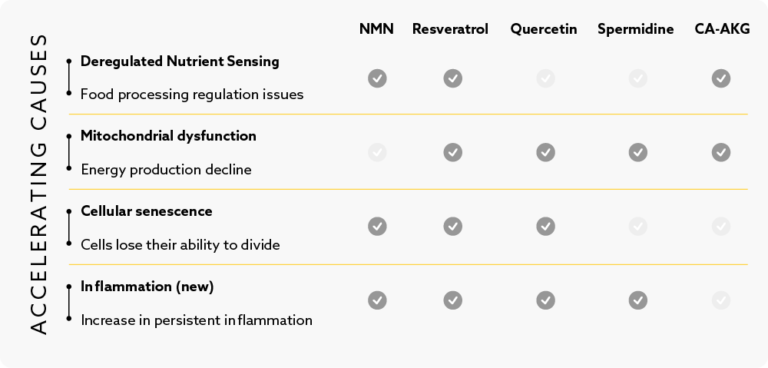
Il rilevamento dei nutrienti è il momento in cui il nostro corpo gestisce l'utilizzo e l'immagazzinamento del cibo che mangiamo. In gioventù, questo sistema funziona in modo efficiente, contribuendo all'energia e alla riparazione delle cellule. Ma con l'avanzare dell'età, può diventare meno preciso. Questo può portare a problemi come l'elaborazione inefficiente degli zuccheri o dei grassi, l'aumento di peso e l'infiammazione. Quando questo sistema è disattivato, può anche interrompere l'autofagia, il processo di pulizia delle nostre cellule. Questa interruzione può accelerare l'invecchiamento e portare a malattie legate all'età.
La disfunzione mitocondriale si riferisce al declino della produzione di energia delle nostre cellule. Quando le cellule dispongono di abbondanti nutrienti, immagazzinano energia. Quando i nutrienti scarseggiano, le cellule si concentrano sulla riparazione. Tuttavia, a volte le cellule non reagiscono correttamente a causa di problemi con le vie di rilevamento dei nutrienti. Alcune proteine svolgono un ruolo nel metabolismo cellulare e nell'invecchiamento. Un modo per affrontare questo problema è ridurre l'apporto calorico dal 10% al 30%, assicurando che i nutrienti essenziali vengano comunque assunti. Questo approccio ha dimostrato di aumentare la salute e la longevità.
I mitocondri, le centrali energetiche delle nostre cellule, producono la maggior parte dell'energia di cui abbiamo bisogno. Ma questo processo rilascia anche particelle nocive chiamate radicali liberi. Nel tempo, queste particelle danneggiano i mitocondri, portando a una produzione di energia meno efficiente, a danni al DNA e a squilibri proteici. Con l'invecchiamento, abbiamo anche meno mitocondri, il che contribuisce alla debolezza muscolare e a un sistema immunitario meno robusto.
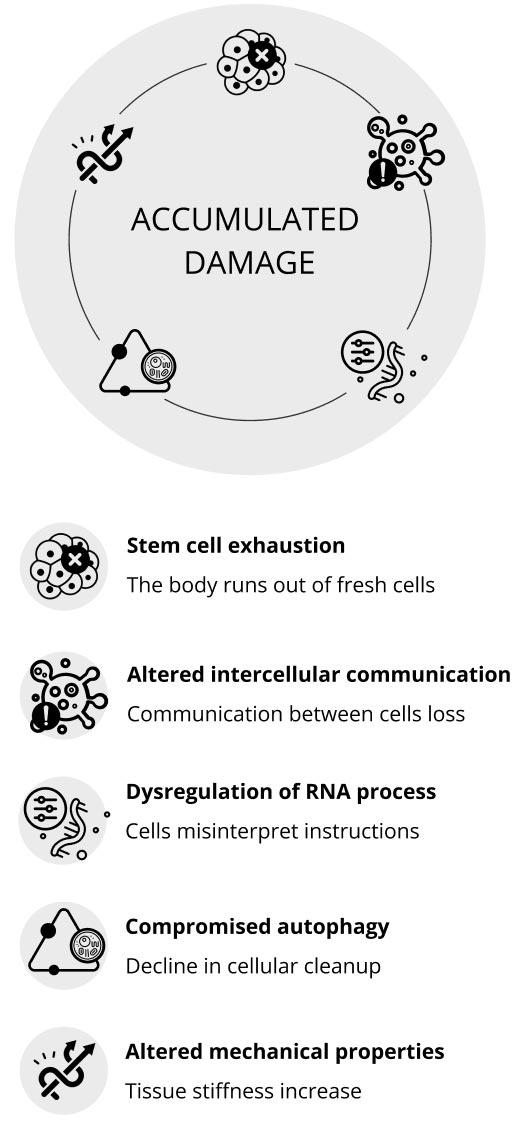
Le cellule senescenti sono cellule vecchie o danneggiate che smettono di dividersi e non aiutano più i tessuti in cui vivono. Queste cellule non solo diventano inattive, ma inviano anche segnali dannosi che possono far diventare senescenti anche le cellule sane. Con il tempo, l'accumulo di queste cellule senescenti può causare problemi al nostro corpo. Possono irrigidire i nostri tessuti, ridurne la funzionalità e portare a malattie legate all'età.
In parole povere, mentre avere alcune cellule vecchie è naturale, averne troppe può accelerare il processo di invecchiamento e causare problemi di salute.
L'infiammazione è un segno chiave dell'invecchiamento, spesso definito "inflammaging". Con l'avanzare dell'età, si può sviluppare un'infiammazione costante e di basso livello, che è collegata a molte malattie legate all'età. I ricercatori ritengono che questo tipo di infiammazione sia così importante da meritare un'attenzione particolare quando si parla di fattori di invecchiamento. L'infiammazione non è isolata, ma può influenzare altri aspetti della nostra salute, tra cui il comportamento delle cellule e l'equilibrio intestinale. Sebbene esistano sostanze come gli estratti di tè verde e lo zenzero che possono aiutare a gestire l'infiammazione, è essenziale comprendere il suo ruolo nel processo di invecchiamento.
Le cellule staminali, fresche e vuote, attendono i segnali per decidere il loro tipo e la loro funzione, il che le rende particolarmente utili per sostituire le cellule danneggiate del nostro corpo. Queste cellule indifferenziate possono trasformarsi in tipi di cellule necessarie e contribuire al mantenimento della salute del nostro corpo.
Tuttavia, le cellule senescenti, vecchie e non funzionanti, emettono segnali che causano infiammazione e possono ridurre l'attività delle cellule staminali, con un impatto sul nostro sistema immunitario e sulla capacità di rigenerazione dei nostri tessuti. Inoltre, problemi come l'accorciamento dei telomeri e cambiamenti genetici possono influire negativamente sulla loro funzionalità nel tempo.
La capacità delle nostre cellule di comunicare tra loro può diminuire nel tempo, portando a ciò che è noto come comunicazione intercellulare alterata. Immaginiamo come dei telefoni con una cattiva ricezione, che causano incomprensioni tra le cellule. Questa "statica" fa sì che le cellule inviino segnali "SOS" quando non dovrebbero. Questi segnali contrastanti possono provocare infiammazioni e un sistema immunitario indebolito, aumentando la nostra vulnerabilità ai problemi di salute.
La disregolazione dello splicing è un segno distintivo dell'invecchiamento che si riferisce all'interruzione del modo in cui le cellule elaborano l'RNA, una molecola vitale per tradurre il nostro codice genetico in proteine funzionali. Lo splicing dell'RNA è come il montaggio di un film, in cui vengono eliminati gli spezzoni non necessari per creare una storia coerente. Con l'invecchiamento, questo processo di "editing" può diventare meno preciso, portando alla produzione di proteine errate o non funzionali. Queste anomalie proteiche possono interrompere le funzioni cellulari, contribuire a comportamenti cellulari anomali e accelerare il processo di invecchiamento. La comprensione della disregolazione dello splicing è fondamentale perché offre spunti di riflessione sulle malattie legate all'età e sui meccanismi più ampi dell'invecchiamento.
La compromissione dell'autofagia è un segno distintivo dell'invecchiamento. Pensiamo all'autofagia come al servizio di pulizia del nostro corpo, responsabile di riordinare e riciclare i componenti cellulari danneggiati. Tuttavia, con il passare del tempo, questo processo di pulizia diventa meno efficace. Questo declino delle pulizie cellulari è legato ai problemi legati all'età, tra cui il declino cognitivo e un sistema immunitario meno reattivo.
Gli studi hanno dimostrato che il potenziamento dell'autofagia può prolungare la durata della vita nei topi e migliorare l'efficacia dei vaccini nelle popolazioni anziane, ringiovanendo la loro risposta immunitaria.
L'invecchiamento si riferisce a cambiamenti nelle cellule e nel loro ambiente circostante. Per esempio, le cellule dei fibroblasti cambiano la loro struttura interna quando invecchiano, il che influisce sul loro movimento e sulla comunicazione con le altre cellule. Questi cambiamenti, soprattutto nel sistema immunitario che invecchia, possono danneggiare i tessuti e nuocere alla salute. Inoltre, il nucleo di una cellula, una parte essenziale, cambia durante l'invecchiamento e può dare il via a ulteriori processi di invecchiamento cellulare. I trattamenti che stabilizzano questa parte della cellula possono prolungare la durata della vita in alcune condizioni che accelerano l'invecchiamento. Inoltre, l'ambiente esterno alla cellula perde flessibilità e diventa più rigido con l'avanzare dell'età, portando a vari problemi e malattie legati all'invecchiamento.


Invecchiamo a causa degli effetti cumulativi dei 12 segni distintivi, come i danni al DNA (instabilità genomica), l'accorciamento dei telomeri e la disfunzione mitocondriale. Questi processi compromettono la riparazione e la funzione delle cellule nel corso del tempo, indotti da fattori genetici, ambientali e dallo stile di vita, portando al declino fisico e alla malattia.
No, l'invecchiamento varia a seconda delle specie. Mentre i mammiferi presentano segni distintivi come l'esaurimento dei telomeri e la senescenza, alcuni organismi (ad esempio alcune meduse o idre) mostrano una senescenza trascurabile, mantenendo il rinnovamento cellulare a tempo indeterminato. Anche tra i mammiferi, i tassi differiscono: i topi invecchiano più velocemente degli esseri umani a causa di variazioni nei meccanismi di riparazione e nel metabolismo.
Sì, lo studio di specie con caratteristiche uniche di invecchiamento, come la senescenza trascurabile delle aragoste o delle idre, può rivelare meccanismi per rallentare l'invecchiamento umano. Ad esempio, la ricerca sui ratti talpa nudi, che resistono al cancro e vivono più a lungo del previsto, evidenzia una maggiore riparazione del DNA e la proteostasi. Queste intuizioni potrebbero essere alla base di interventi che mirano a contrastare caratteristiche come l'instabilità genomica o la senescenza cellulare, portando potenzialmente a terapie in grado di prolungare la durata della salute umana.
I segni distintivi dell'invecchiamento sono 12 processi biologici, come l'instabilità genomica, il logorio dei telomeri e la disfunzione mitocondriale, che determinano il declino e le malattie legate all'età. Essi interagiscono per accelerare l'invecchiamento.
Questi segni distintivi causano danni alle cellule e ai tessuti, portando a malattie come il cancro, l'Alzheimer e le malattie cardiache, riducendo al contempo la vitalità generale e la durata della vita.
Sebbene non siano completamente reversibili, interventi come l'esercizio fisico, la restrizione calorica e gli integratori (ad esempio l'NMN) possono rallentare o attenuare alcuni segni distintivi, come la senescenza cellulare o la disfunzione mitocondriale.
L'infiammazione cronica, o "inflammaging", danneggia i tessuti e promuove malattie come l'artrite e il diabete interrompendo la comunicazione cellulare e amplificando altri segni distintivi.
Una dieta equilibrata, ricca di antiossidanti e nutrienti, supporta la proteostasi, riduce l'infiammazione e stimola l'autofagia, aiutando a contrastare i processi di invecchiamento.
L'NMN aumenta i livelli di NAD+, supportando i percorsi di rilevamento dei nutrienti e la funzione mitocondriale, che può rallentare segni distintivi come il rilevamento deregolato dei nutrienti e il declino energetico.
L'esercizio fisico regolare, la gestione dello stress, il sonno di qualità e il digiuno intermittente possono potenziare l'autofagia, ridurre la senescenza e migliorare la funzione delle cellule staminali per promuovere un invecchiamento sano.
Siamo qui per rendere la vita sana senza sforzo. I cookie aiutano il nostro sito web a funzionare al meglio e ci permettono di capire come migliorare la tua esperienza con Purovitalis. Informativa sulla privacy - Imprint
Questi cookie permettono al sito di funzionare, come il carrello della spesa, il checkout e le impostazioni di sicurezza. Senza di essi, il sito non potrebbe funzionare correttamente.
Questi ci aiutano a ricordare le tue scelte e a migliorare la tua esperienza, ad esempio mostrandoti la lingua giusta o le domande del sondaggio più adatte ai tuoi interessi.
Questi cookie ci mostrano come i visitatori utilizzano il nostro sito in modo da poterlo rendere più veloce, chiaro e utile. Tutti i dati vengono raccolti in forma anonima.
Questi ci aiutano a condividere offerte e informazioni rilevanti su Purovitalis su altri siti e piattaforme, in modo che tu possa vedere i contenuti che ti interessano.








I piani Start includono 3 consegne al mese e poi continuano ogni mese a meno che non li disdici.
Prima di 3 consegne
Puoi annullare in qualsiasi momento, ma il piano rimarrà attivo fino a quando tutte e 3 le consegne mensili non saranno completate.
Dopo 3 consegne
Puoi cancellare in qualsiasi momento prima del prossimo rinnovo. Il piano non finisce da solo e devi cancellarlo a mano.
Messa in pausa o riprogrammazione
Puoi mettere in pausa o cambiare la data di consegna in qualsiasi momento dal tuo account.
Resi e rimborsi
Gli ordini in abbonamento non possono essere rimborsati una volta spediti. I prodotti già consegnati come parte di un piano Start non possono essere restituiti.
Puoi gestire il tuo piano in qualsiasi momento da Il mio account → Abbonamenti, oppure scrivici a [email protected].
Registrati per una prova gratuita di 7 giorni della nostra applicazione con intelligenza artificiale e fai il primo passo verso una persona più giovane e più sana.
oppure ottieni l'accesso completo con l'abbonamento al prodotto!
La Prof.ssa Andrea Maier è internista e docente di invecchiamento ("medicina della longevità") presso la Vrije Universiteit di Amsterdam e l'Università di Melbourne, in Australia. Studia l'invecchiamento del corpo e ricerca trattamenti anti-invecchiamento. Dirige il Center for Healthy Longevity di Singapore.
Perché il nostro corpo invecchia gradualmente durante la nostra vita media di oltre 80 anni? Possiamo fermare questo processo? O forse addirittura invertire la rotta? E fino a che punto dovremmo volerlo? Maier fornisce consigli pratici su come allungare la durata della nostra vita rimanendo in salute.
Andrea Maier si è laureata in Medicina all'Università di Lubecca nel 2003. Si è specializzata in medicina interna presso il Centro Medico dell'Università di Leida e successivamente ha scelto la sottospecialità di Medicina Geriatrica. È qui che ha iniziato la sua ricerca sull'invecchiamento.
Vantaggi esclusivi
Convenienza
Coerenza
Risparmio di tempo